
-

生物通官微
陪你抓住生命科技
跳动的脉搏
农药团队在核酸农药创制方面取得新进展
【字体: 大 中 小 】 时间:2023年10月10日 来源:华中农业大学植物科学技术学院
编辑推荐:
南湖新闻网讯(通讯员 吕海翔 于畅)近日,植物科学技术学院李建洪教授领衔的农药毒理学与有害生物抗药性团队分别在Chemical Engineering Journal和ACS Applied Materials & Interfaces发表了题为“Overcoming resistance in insect pest with a nanoparticle-mediated dsRNA and insecticide co-delivery system”和“Metal−Organic Framework-Based Insecticide and dsRNA Codelivery System for Insecticide Resistance Management”的研究论文,报道了两种基于RNAi技术的害虫抗药性治理方法,研究采用dsRNA与杀虫剂共递送的方式,突破了害虫抗药性治理和新农药创制的传统思路,为我国棉花、水稻等作物杀虫剂减量使用和绿色高质量安全生产提供了新途径
南湖新闻网讯(通讯员 吕海翔 于畅)近日,植物科学技术学院李建洪教授领衔的农药毒理学与有害生物抗药性团队分别在Chemical Engineering Journal和ACS Applied Materials & Interfaces发表了题为“Overcoming resistance in insect pest with a nanoparticle-mediated dsRNA and insecticide co-delivery system”和“Metal−Organic Framework-Based Insecticide and dsRNA Codelivery System for Insecticide Resistance Management”的研究论文,报道了两种基于RNAi技术的害虫抗药性治理方法,研究采用dsRNA与杀虫剂共递送的方式,突破了害虫抗药性治理和新农药创制的传统思路,为我国棉花、水稻等作物杀虫剂减量使用和绿色高质量安全生产提供了新途径。
基于RNAi技术的害虫抗药性治理是一种极具潜力的有害生物防治策略。然而,dsRNA在环境中的不稳定性使其无法直接应用于农业生产。为解决这一问题,团队采用纳米载体同时负载杀虫剂和dsRNA,以害虫关键抗性基因为靶标,实现了害虫的绿色高效防控。纳米载体可以显著提升dsRNA的环境稳定性,并实现杀虫剂和外源dsRNA在植物和害虫体内的高效递送,显著提高了杀虫剂对害虫的生物活性。研究结果为抗性害虫治理提供了新的思路,在有害生物绿色高效防控方面具有巨大应用潜力。
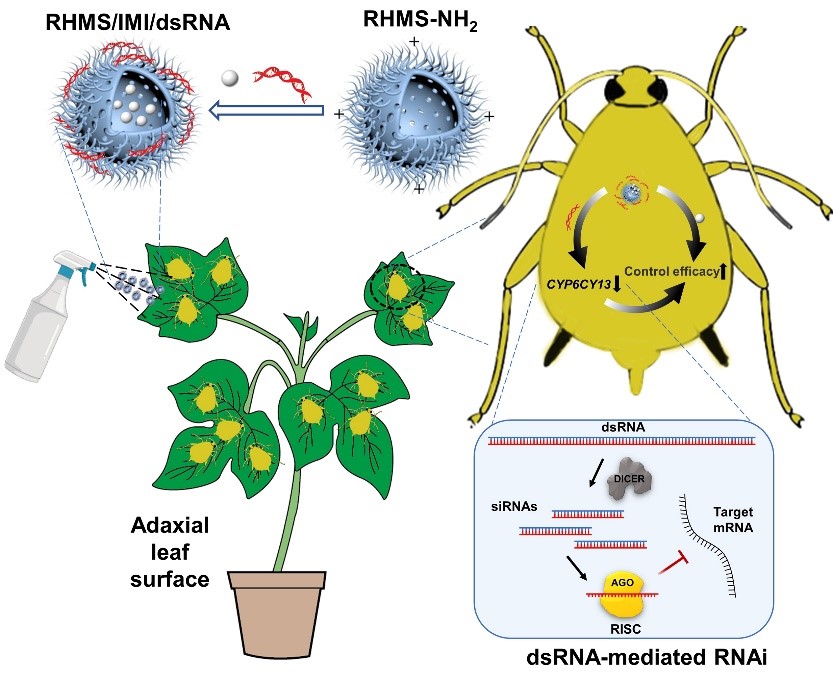
图1棉蚜体壁渗透型共递送纳米农药的制备及作用机制
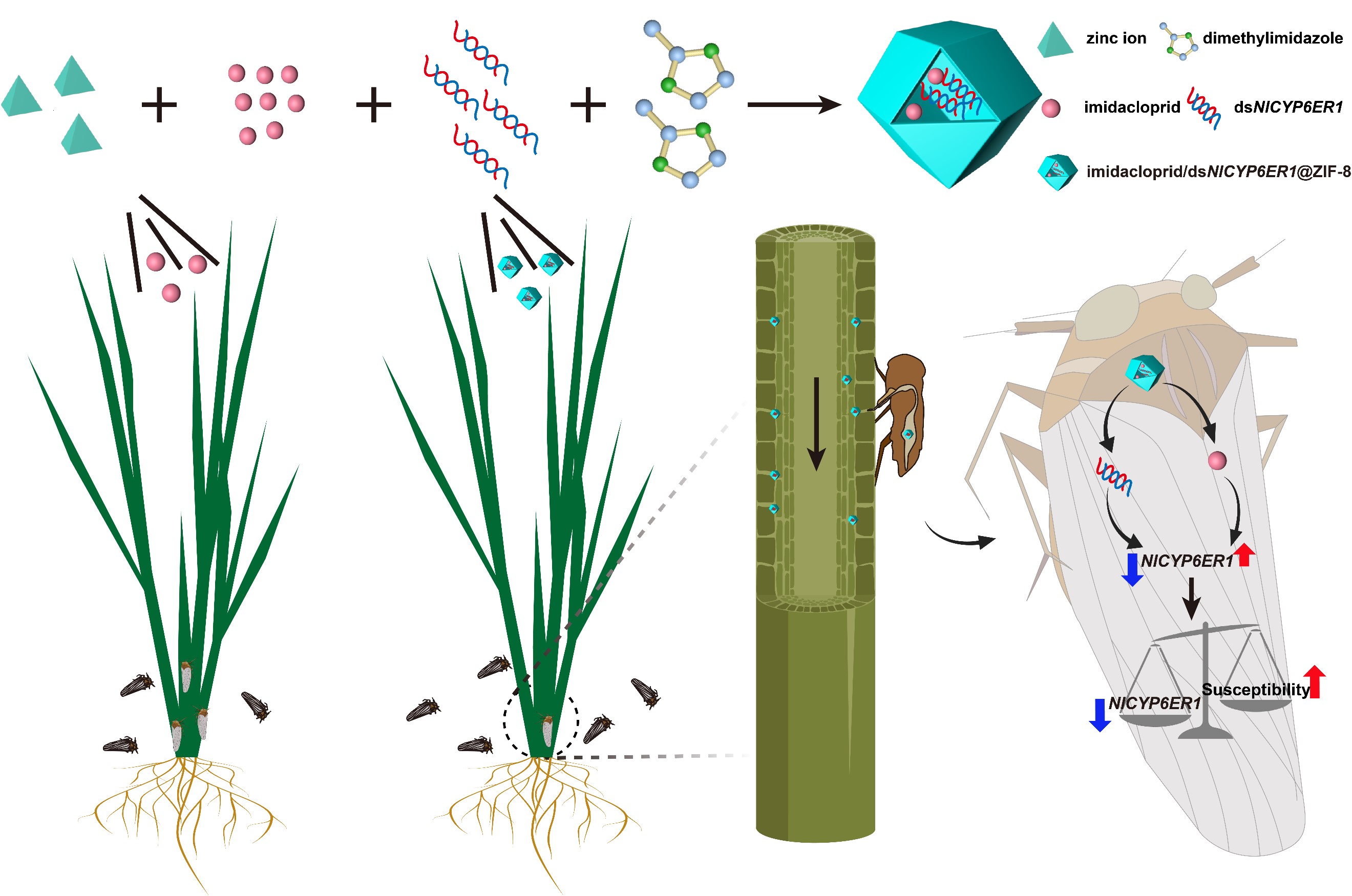
图2 imidacloprid/dsNlCYP6ER1@ZIF-8RNAi纳米农药的制备及作用机制
基于共同递送杀虫剂和dsRNA的策略,构建了基于表面粗糙中空介孔二氧化硅(RHMS)与沸石型咪唑盐框架8(ZIF-8)纳米载体的RHMS/IMI/dsCYP6CY13(图1)与imidacloprid/dsNlCYP6ER1@ZIF-8(图2)共递送系统。RHMS利用其中空介孔结构高效负载杀虫剂,并进一步通过静电作用与dsRNA结合形成纳米级复合物,保护dsRNA免受核酸酶降解。由于RHMS/IMI/dsCYP6CY13具有独特的结构,可穿透棉蚜(Aphis gossypii)体壁,将杀虫剂与dsRNA共同递送至棉蚜体内,抑制关键抗性基因CYP6CY13的表达,提高了杀虫剂的杀虫效果。imidacloprid/dsNlCYP6ER1@ZIF-8共递送系统具有均一的粒径和良好的分散性,能够将吡虫啉与dsRNA高效递送至水稻茎基部,褐飞虱(Nilaparvata lugens)取食后,抑制抗吡虫啉褐飞虱关键抗性基因(NlCYP6ER1)的表达,从而提高了褐飞虱对吡虫啉的敏感性。
植物科学技术学院博士研究生吕海翔和于畅分别为论文第一作者,何顺副教授和马康生副教授为论文的通讯作者,李建洪教授和万虎教授参与了相关研究工作,以上研究得到了国家重点研发计划、湖北省重点研发计划和国家自然科学基金等项目的资助。
审核人:李建洪
【英文摘要1】
The cotton aphid Aphis gossypii Glover (Homoptera: Aphididae) is a notorious agricultural pest that has evolved high levels of resistance to various insecticides. Innovative RNA insecticides, as potential green plant protection products, are anticipated to alleviate the significant issue of pest resistance. Herein, a rough-surface hollow mesoporous silica (RHMS)-based gene/insecticide delivery system was constructed for the co-delivery of CYP6CY13 double-stranded RNA (dsRNA) and the chemical insecticide imidacloprid for the effective control of A. gossypii. The hollow mesoporous structure of RHMS facilitates insecticide loading and further combines with dsRNA through electrostatic interactions to form nanoscale complexes, while protecting the dsRNA from complete degradation by nucleases. The screened dsCYP6CY13 fragment was successfully expressed in the L4440-HT1115 (DE3) RNaseIII- system and inhibiting CYP6CY13 expression by RNA interference (RNAi) significantly increased the susceptibility of A. gossypii adults to imidacloprid. The toxicity of the RHMS/IMI/dsCYP6CY13 complex was significantly higher (1.95-fold) than that of imidacloprid. The results of the pot test showed that the effectiveness of the RHMS/IMI/dsCYP6CY13 complex against A. gossypii improved by 19.95 % in 5 days. Therefore, this nanoparticle-mediated gene/insecticide co-delivery system is expected to be an effective tool for pest control.
论文链接1:https://www.sciencedirect.com/science/article/pii/S1385894723049707
【英文摘要2】
Targeted silencing of resistance-associated genes by specific double-stranded RNA (dsRNA) is an attractive strategy for overcoming insecticide resistance in insect pests. However, silencing target genes of insect pests by feeding on dsRNA transported via plants remains challenging. Herein, a codelivery system of insecticide and dsRNA is designed by encapsulating imidacloprid and dsNlCYP6ER1 within zeolitic imidazolate framework-8 (ZIF-8) nanoparticles to improve the susceptibility of Nilaparvata lugens (Stål) to imidacloprid. With an average particle size of 195 nm and a positive surface charge, the derived imidacloprid/dsNlCYP6ER1@ZIF-8 demonstrates good monodispersity. Survival curve results showed that the survival rates of N. lugens treated with imidacloprid and imidacloprid@ZIF-8 were 82 and 62%, respectively, whereas, in the imidacloprid/dsNlCYP6ER1@ZIF-8 treatment group, the survival rate of N. lugens is only 8%. Pot experiments demonstrate that the survival rate in the imidacloprid/dsNlCYP6ER1@ZIF-8 treatment group was much lower than that in the imidacloprid treatment group, decreasing from 54 to 24%. The identification of NlCYP6ER1 expression and the fluorescence tracking of ZIF-8 demonstrate that ZIF-8 can codeliver dsRNA and insecticide to insects via rice. Safety evaluation results showed that the dsNlCYP6ER1@ZIF-8 nanoparticle had desirable biocompatibility and biosafety to silkworm. This dsRNA and insecticide codelivery system may be extended to additional insecticides with potential resistance problems in the future, greatly enhancing the development of pest resistance management.